The complexity of energy homeostasis regulation involves multiple overlapping hormonal systems, each contributing to the integrated control of appetite, glucose metabolism, and body composition. Cagrilintide, a long-acting amylin analog, represents a novel approach to metabolic research that complements established incretin-based strategies. This analysis examines the amylin pathway, its synergistic potential with GIP and GLP-1 signaling, and the implications for obesity research paradigms.
Amylin: The Often-Overlooked Satiety Hormone
Amylin, also known as islet amyloid polypeptide (IAPP), is a 37-amino acid peptide hormone co-secreted with insulin from pancreatic β-cells in response to nutrient ingestion. Despite its discovery in the late 1980s, amylin has received considerably less research attention than the incretins, yet plays important and distinct roles in metabolic regulation.
The physiological effects of amylin include:
- Satiety signaling: Acts on area postrema and hypothalamic nuclei to reduce food intake
- Gastric emptying: Slows gastric motility, prolonging nutrient contact with intestinal sensors
- Glucagon suppression: Inhibits postprandial glucagon secretion, reducing hepatic glucose output
- Postprandial glucose: Contributes to glucose excursion blunting through multiple mechanisms
Critically, amylin’s satiety effects are mediated through receptors and pathways distinct from GLP-1, suggesting potential for additive or synergistic effects when both systems are engaged simultaneously.
Amylin Receptor Pharmacology
Receptor Complex Architecture
The amylin receptor system exemplifies the complexity of peptide hormone signaling. Rather than a single dedicated receptor, amylin signals through heteromeric complexes formed by the calcitonin receptor (CTR) with receptor activity-modifying proteins (RAMPs).
Three primary amylin receptor subtypes exist:
- AMY1: CTR + RAMP1 (highest amylin affinity)
- AMY2: CTR + RAMP2
- AMY3: CTR + RAMP3
This receptor architecture creates pharmacological complexity, as different tissues express varying combinations of CTR and RAMPs, potentially enabling tissue-selective effects with appropriately designed ligands.
Signal Transduction
Amylin receptor activation primarily couples to Gαs, elevating intracellular cAMP—similar to GLP-1R signaling. However, differences in receptor distribution, coupling efficiency, and downstream pathway engagement create distinct physiological effects:
- Area postrema: High receptor expression enables circulating amylin to access CNS satiety circuits
- Hypothalamus: Modulation of energy balance neurons
- Brainstem: Integration with vagal satiety signals
Cagrilintide: Long-Acting Amylin Analog
Structural Modifications
Native amylin presents challenges for research and therapeutic application due to its tendency to aggregate and form amyloid fibrils—the same process implicated in islet amyloid deposits in type 2 diabetes. Additionally, the peptide has a short plasma half-life of approximately 13 minutes.
Cagrilintide addresses these limitations through strategic modifications:
- Sequence modifications: Amino acid substitutions reduce aggregation propensity
- Acylation: Fatty acid attachment (similar to semaglutide) enables albumin binding and half-life extension
- Result: Once-weekly dosing feasibility with stable, non-aggregating formulation
“The development of Cagrilintide represents a significant advance in amylin pharmacology. By engineering a stable, long-acting analog, researchers can now investigate sustained amylin receptor engagement in ways previously impossible with the aggregation-prone native peptide.” — Metabolic Peptide Research Review, 2023
Pharmacokinetic Profile
Cagrilintide achieves a plasma half-life of approximately one week, enabling once-weekly subcutaneous administration in research protocols. This extended profile provides sustained amylin receptor engagement, contrasting with the pulsatile exposure achieved with native amylin or shorter-acting analogs like pramlintide.
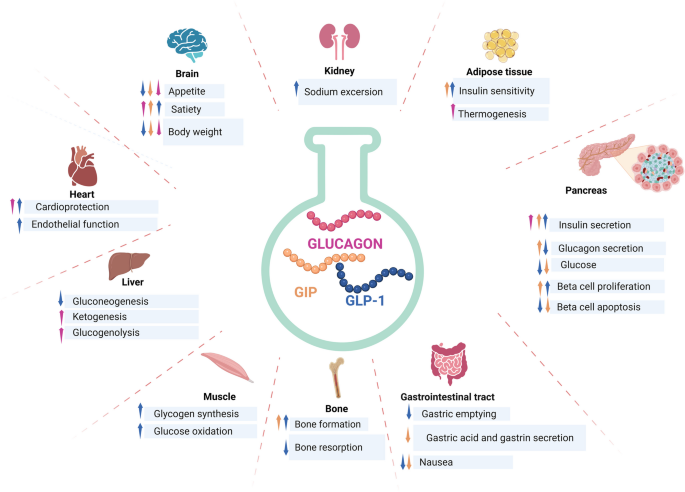
GIP: The “Other” Incretin
GIP Physiology
Glucose-dependent insulinotropic polypeptide (GIP), formerly called gastric inhibitory polypeptide, is secreted by K-cells in the proximal small intestine in response to nutrient ingestion. As an incretin, GIP potentiates glucose-dependent insulin secretion from pancreatic β-cells.
GIP has historically been viewed as less therapeutically promising than GLP-1 due to observations of GIP resistance in type 2 diabetes. However, recent research has renewed interest in GIP for several reasons:
- Adipose tissue effects: GIP receptors on adipocytes influence lipid metabolism
- Bone effects: GIP promotes bone formation and may prevent bone loss
- CNS effects: Emerging evidence for central GIP signaling in energy balance
- Synergy with GLP-1: Combination approaches show enhanced efficacy
GIP Receptor Distribution
The GIP receptor (GIPR), a Class B GPCR like GLP-1R, is expressed in:
- Pancreatic islets: β-cells (insulin secretion) and α-cells
- Adipose tissue: Both white and brown adipocytes
- Bone: Osteoblasts and osteoclasts
- Brain: Multiple regions including hypothalamus
- Cardiovascular system: Heart and blood vessels
Rationale for Multi-Receptor Targeting
Complementary Mechanisms
The rationale for combining amylin agonism with incretin receptor engagement derives from their complementary but non-overlapping mechanisms:
| Pathway | Primary Site | Key Effects |
|---|---|---|
| Amylin | Area postrema, Hypothalamus | Satiety, Gastric emptying, Glucagon suppression |
| GLP-1 | Pancreas, Brain, Gut | Insulin secretion, Satiety, Gastric emptying |
| GIP | Pancreas, Adipose, Bone | Insulin secretion, Lipid metabolism, Bone health |
By engaging multiple pathways simultaneously, combination approaches may achieve effects greater than the sum of individual components—true pharmacological synergy.
CagriSema: The Combination Approach
Research into the combination of Cagrilintide with Semaglutide (termed “CagriSema”) exemplifies the multi-target strategy. Early-phase studies have demonstrated weight reduction exceeding that achieved with either component alone, supporting the synergy hypothesis.
The mechanistic basis for synergy likely includes:
- Convergent satiety pathways: Amylin and GLP-1 activate distinct CNS circuits that integrate to enhance overall satiety
- Complementary kinetics: Different time courses of receptor activation may prevent adaptation
- Peripheral effects: GLP-1’s pancreatic and metabolic effects complement amylin’s postprandial glucose effects
Adipose Tissue as a Target
GIP Effects on Adipocytes
The GIP receptor is well-expressed on adipocytes, and GIP signaling influences multiple aspects of adipose biology:
- Lipogenesis: GIP promotes fatty acid uptake and triglyceride synthesis
- Lipoprotein lipase: Enhanced activity facilitates lipid storage
- Insulin sensitivity: May improve adipocyte insulin signaling
- Adipokine secretion: Potential modulation of adiponectin and other factors
Paradoxically, this apparently “pro-lipogenic” profile may contribute to metabolic benefits by promoting appropriate nutrient partitioning to adipose tissue rather than ectopic lipid accumulation in liver and muscle.
Amylin and Adipose Tissue
Amylin’s effects on adipose tissue are less well-characterized than GIP’s, but evidence suggests:
- Possible direct effects on adipocyte metabolism through local receptor expression
- Indirect effects through CNS-mediated changes in sympathetic tone
- Modulation of postprandial substrate flux affecting adipose storage
Research Applications
Metabolic Phenotyping Studies
The availability of long-acting amylin analogs enables research into sustained amylin receptor engagement effects:
- Energy expenditure: Indirect calorimetry studies with chronic amylin exposure
- Body composition: DXA or MRI assessment of fat and lean mass changes
- Glucose homeostasis: Clamp studies, oral glucose tolerance tests
- Lipid metabolism: Plasma lipid profiles, hepatic fat content
Combination Protocol Design
Researchers investigating multi-target approaches must consider several factors:
- Dose ratios: Optimal relative dosing of each component may differ from single-agent studies
- Administration timing: Simultaneous versus staggered dosing
- Endpoint selection: Weight, glycemia, food intake, and mechanistic markers
- Duration: Acute effects versus steady-state metabolic adaptation
Mechanistic Investigation
Understanding the basis for combination synergy requires detailed mechanistic studies:
- CNS studies: c-Fos mapping, electrophysiology, microdialysis
- Receptor dynamics: Internalization, desensitization, cross-talk
- Signaling integration: How do amylin and incretin pathways converge?
- Tissue-specific effects: Which tissues drive the synergistic response?
Challenges and Considerations
Receptor Cross-Talk
When multiple receptor systems are engaged simultaneously, complex cross-talk phenomena may emerge. The amylin receptor shares structural features with the calcitonin receptor family, and potential interactions with the GLP-1 and GIP systems at the level of intracellular signaling merit consideration.
Tolerability Profiles
Gastrointestinal effects (nausea, reduced appetite) are common with both amylin and GLP-1 receptor agonists. Combination approaches may require careful dose titration to manage these effects while achieving metabolic benefits.
Individual Variability
Response to incretin and amylin-based interventions varies substantially between individuals. Understanding the determinants of this variability—genetic, metabolic, microbiome-related—represents an important research frontier.
Quality Considerations for Research
For rigorous investigation of multi-target metabolic approaches, peptide quality is paramount:
- Purity (HPLC): ≥98% ensures minimal interference from synthesis impurities
- Identity (MS): Mass spectrometry confirmation of correct sequence and modifications
- Aggregation testing: Particularly important for amylin-related peptides
- Stability: Appropriate storage to maintain activity
- Endotoxin: Low levels for in vivo applications
Future Directions
The field of multi-receptor metabolic targeting continues to evolve:
- Triple agonists: Compounds engaging GLP-1, GIP, and glucagon receptors simultaneously
- Quadruple targeting: Adding amylin agonism to triple combinations
- Biased agonism: Pathway-selective ligands for each receptor system
- Oral delivery: Enabling non-injectable administration of peptide combinations
- Personalized approaches: Tailoring receptor targeting to individual metabolic profiles
Conclusion
The convergence of amylin and incretin research represents an exciting frontier in metabolic science. Cagrilintide’s development as a stable, long-acting amylin analog enables investigation of sustained amylin receptor engagement, while GIP’s emerging importance adds another dimension to multi-receptor strategies.
The synergistic potential of combining these pathways—engaging complementary but distinct mechanisms of appetite control, glucose homeostasis, and energy balance—offers possibilities exceeding what any single-receptor approach might achieve. As research tools become available and mechanistic understanding deepens, the rational design of multi-target metabolic interventions becomes increasingly feasible.
Regenpep provides research-grade peptides for metabolic research with comprehensive quality documentation. Our commitment to purity and analytical verification supports rigorous investigation of these complex signaling systems.